Abstract
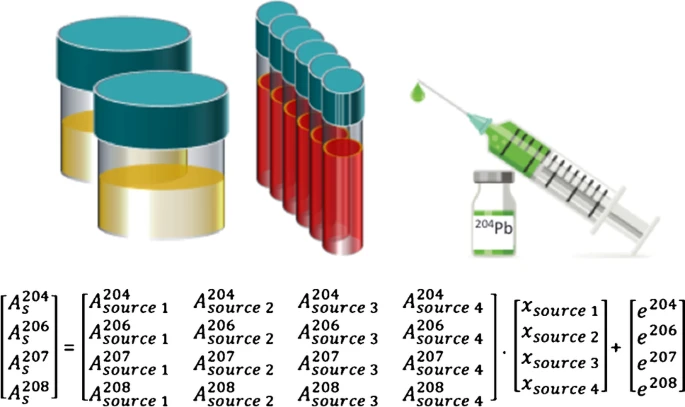
The potential of enriched Pb (204Pb) was assessed to monitor pathways of trace levels of Pb in the pg range within the human body via isotope pattern variation in situations where natural lead cannot be used as a tracer due to regulatory limitations. Isotope ratio measurements were accomplished by means of (multi-collector) inductively coupled plasma mass spectrometry including a comparison of single and multi-collector ICP-MS for low-level 204Pb assessment. Isotopic pattern results from a blend of a large quantity of the element with a natural isotopic composition and an enriched stable isotope at orders of magnitude lower levels pose a nontrivial analytical problem. Isotope pattern deconvolution was successfully applied as mathematical tool based on multiple linear regressions.
The method allowed for deconvolving the isotope pattern from measured isotope ratios without knowing the quantities of different isotope sources incorporated and mixed into the sample at levels of<1 pg 204Pb/g blood. The objective of this manuscript is to evaluate and summarize the analytical aspects for Pb isotope pattern deconvolution based on the results of a clinical trial, where a 204Pb-enriched isotope tracer was applied to investigate the bioavailability of orally applied Pb along with purified clinoptilolite tuff as potential supplement. This unique approach allows to reduce tracer amounts to harmless levels to human health, which are in accordance with the legal regulative to study enrichment levels of < 0.01% in human blood.
https://link.springer.com/article/10.1007/s00216-022-04311-0